
- Improve governance and financial sustainability;
- Optimize surveillance systems;
- Human capacity development;
- Supporting the use of technology;
- Monitoring of Adverse Reactions Reporting.
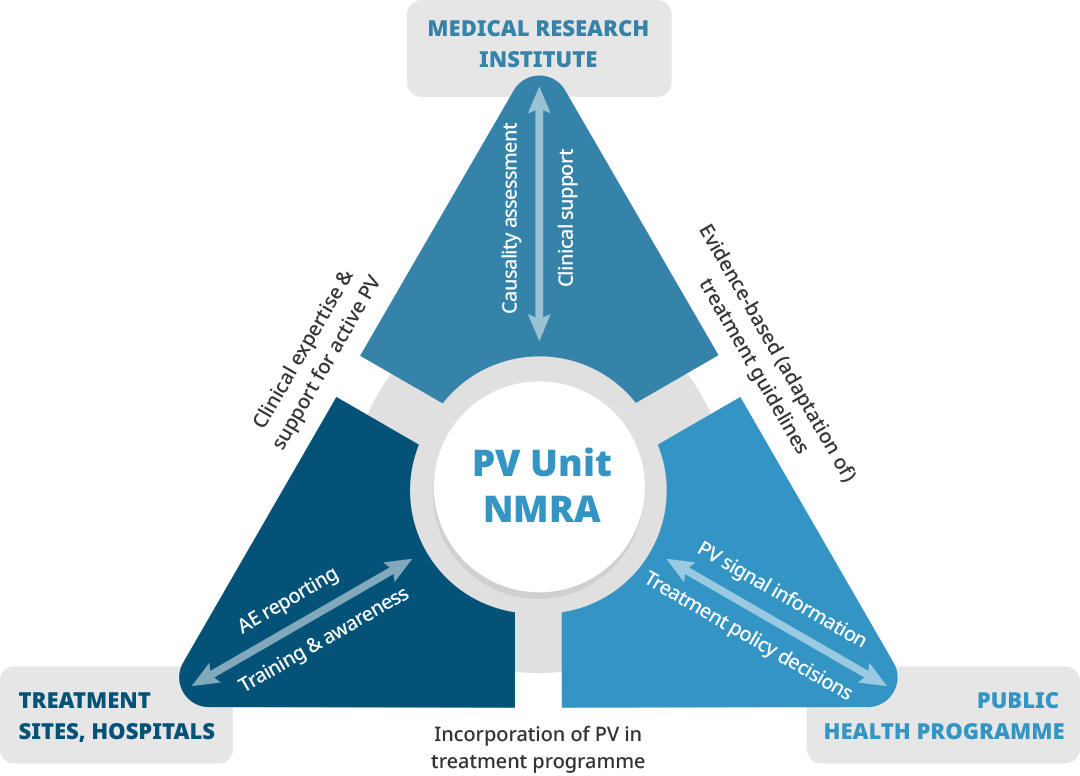
PAVIA's focus was on strengthening routine adverse event reporting, providing guidance on causality assessment and signal detection, and training at national and lower levels to support the linkage process between disease control programs and regulatory pharmacovigilance agencies.
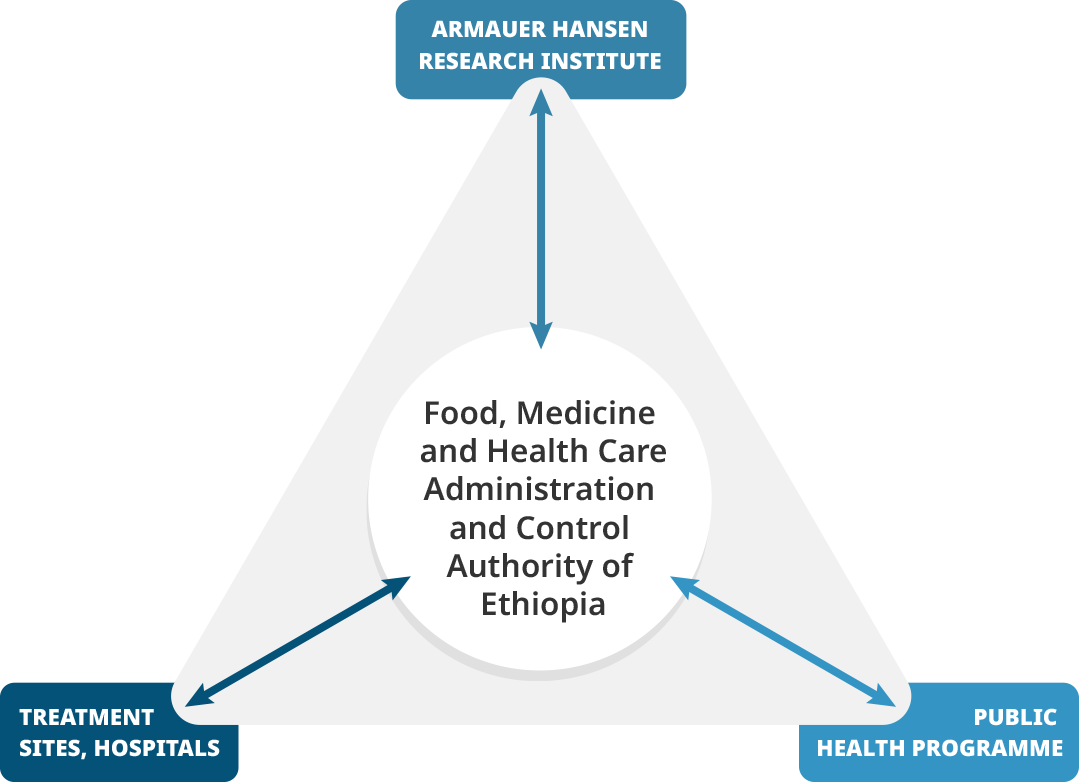
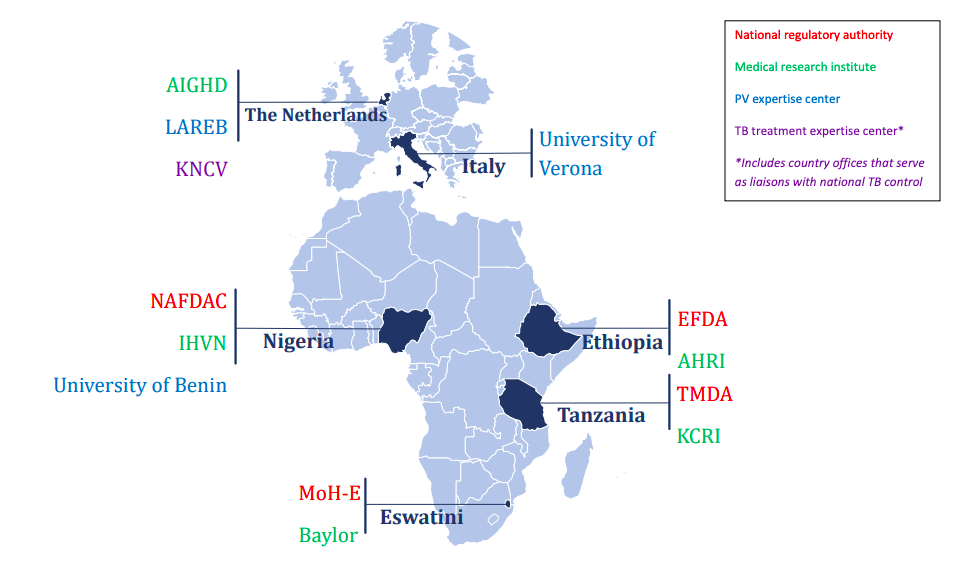
In Ethiopia, the Food, Medicine and Healthcare Administration and Control Authority (FMHACA) hosted one pharmacovigilance head office. The Armauer Hansen Research Institute (AHRI) was a leading MRI working on various product requirements documents, including tuberculosis and local capacity building for operational research.
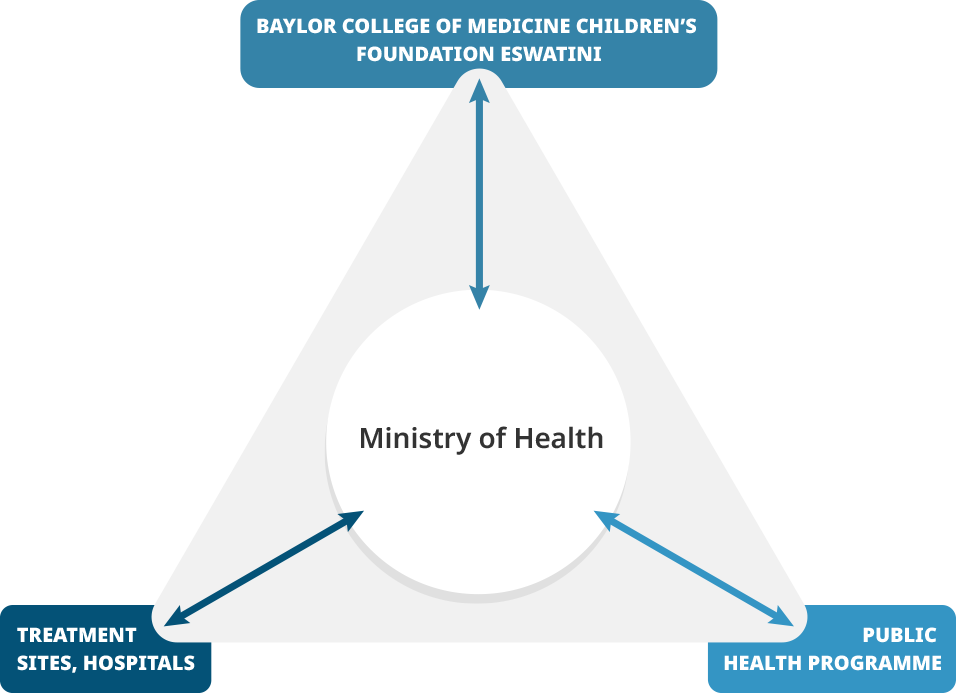
In Eswatini (former Swaziland), the Ministry of Health Swaziland (MoH-S) had been delegated responsibility for PV activities, especially on HIV and tuberculosis. MoH-S brought expertise on PV/PHP collaboration models. They worked in close collaboration with the Baylor College of Medicine Swaziland.
 |
 |
|
Ethiopia Triangle |
Eswatini Triangle |
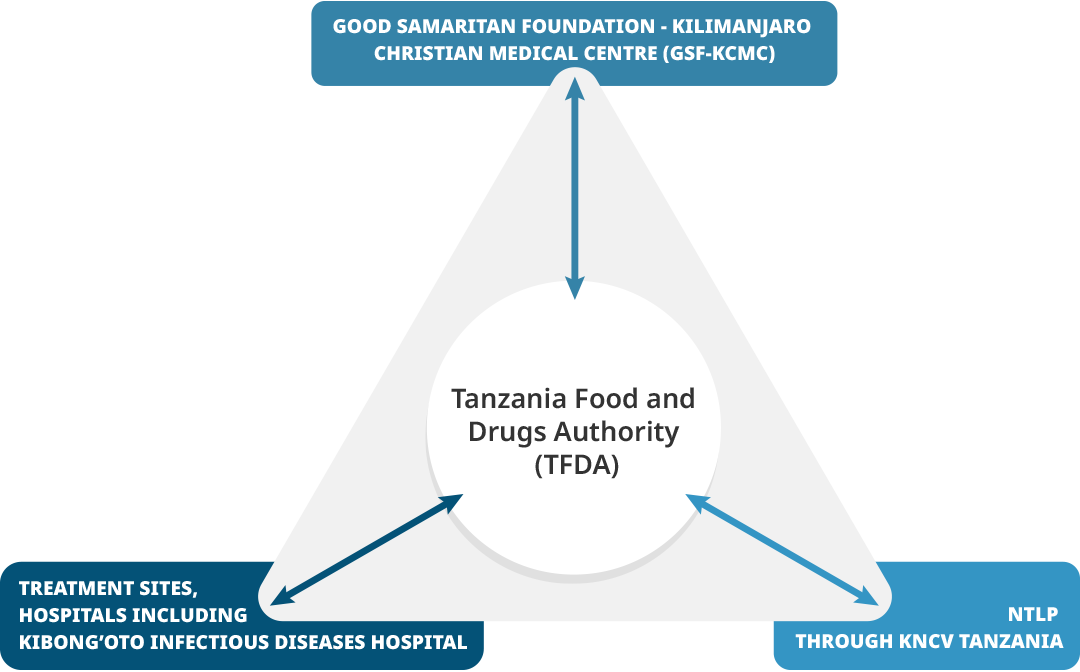
In Tanzania, the national pharmacovigilance center was hosted by the Tanzania Medicines and Medical Devices Authority (TMDA), which had seven zonal offices and seven pharmacovigilance centers at referral hospitals. Kilimanjaro Clinical Research Institute (KCRI) provided research training and infrastructure for investigators and partners at Kilimanjaro Christian Medical Centre (KCMC) and had implemented clinical trials for tuberculosis treatment, including MDR-TB.
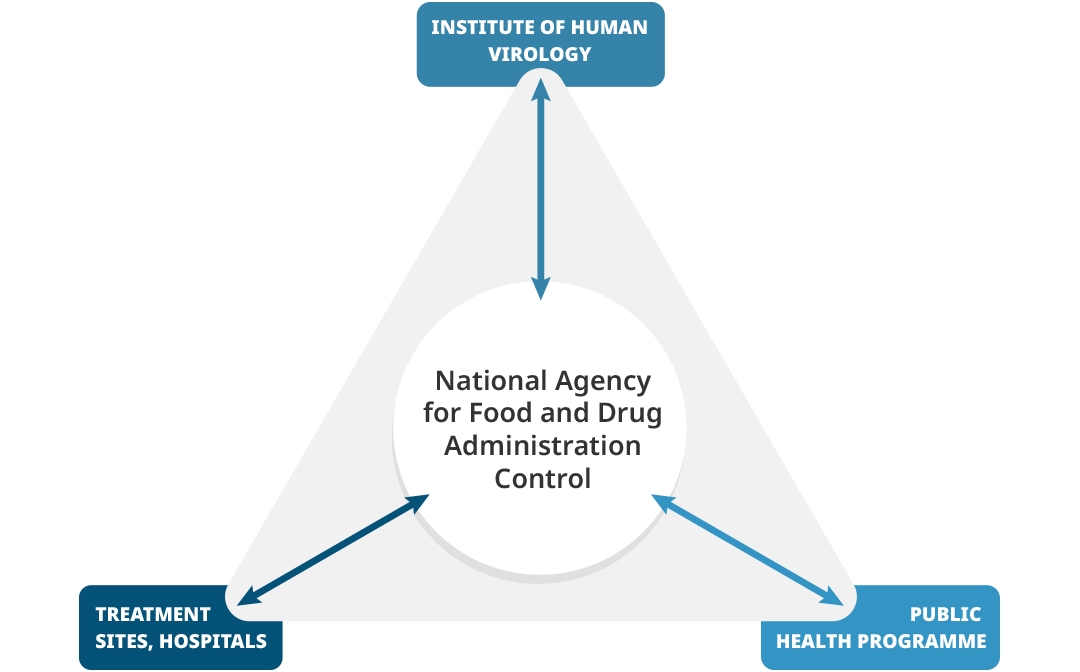
In Nigeria, the National Agency for Food & Drug Administration and Control (NAFDAC) is the host of the national pharmacovigilance center and worked closely with the Institute of Human Virology Nigeria (IHVN) to train healthcare professionals in detecting, reporting, and monitoring adverse drug reactions (ADR) for HIV/AIDS, tuberculosis, and malaria. The University of Benin (UNIBEN) in Nigeria coordinated and supported NAFDAC, TDFA, FMHACA, and MoH-S to strengthen pharmacovigilance policies and legislation and ensured high-level engagement with the Ministry of Health in each country. A harmonized legislation on medicines regulation in African countries was developed, supporting the African Union's goal of promoting local pharmaceutical production for the protection of public health.
 |
 |
|
Tanzania Triangle |
Nigeria Triangle |
The project was coordinated by the Amsterdam Institute for Global Health and Development, the Netherlands. The University of Verona, Italy, supported in building the eLearning modules and hosting the trainings. Netherlands Pharmacovigilance Centre Lareb provided the project with valuable insight in how to Improve pharmacovigilance processes, tools and technologies and oversaw the project’s data collection and signal detection, including data management measures. The KNCV Tuberculosis Foundation, the Netherlands, supported the project by analyzing the existing pharmacovigilance structures and processes at baseline, developing country-specific roadmaps, evaluating the impact of the project on its outputs, and by developing the blueprint document that can be used to scale-up pharmacovigilance in other African countries.
Resources:
The PAVIA project aims to strengthen pharmacovigilance (PV) in four African countries: Ethiopia, Eswatini, Nigeria, and Tanzania. To achieve this aim, collaborative support will be harnessed across several institutions in Europe and Africa.
Our Partners:
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
